Introduction to Stereotactic Radiosurgery
![]()
The development of stereotactic radiosurgery (SRS) has been a long process that involved contributions from a multitude of disciplines and imaginative clinicians, physicists, and engineers. This innovation has enabled millions of patients to recover from life-threatening tumors. Unlike typical surgery, there’s no incision necessary for this treatment. Instead, SRS uses precise imaging to target high doses of radiation to the affected area with minimal impact on the surrounding healthy tissue. In this blog, the development of SRS will be covered. Additionally, we will discuss the mechanisms of how this treatment works, what types of malignancies can benefit from SRS and the different types of machinery that can perform this procedure.
History of Development
Stereotactic radiosurgery was first developed in 1949 by the Swedish neurosurgeon Lars Leksell to treat small targets in the brain that were not amenable to conventional surgery. Leksell envisioned a treatment that would be a less invasive and safer alternative to standard brain surgery which requires incisions in the skin, skull, and membranes surrounding the brain. The initial stereotactic instrument he conceived used probes and electrodes; however, this was quickly replaced with radiation in the form of x-rays. Leksell proceeded to develop a practical, tool that could be handled by the surgeon himself. In 1968 this resulted in the Gamma Knife, which was installed at the Karolinska Institute and consisted of several cobalt-60 radioactive sources placed in a helmet with central channels for irradiation with gamma rays. Improvements of the first Gamma knife led to the development and installment of a unit with 201 cobalt-60 sources in the 1980s.
At the same time as these developments, a similar approach was designed for a linear particle accelerator (Linac). Installation of the first 4 MeV clinical linear accelerator began in June 1952 in the Medical Research Council Radiotherapeutic Research Unit at the Hammersmith Hospital, London. Extensive trials and quality control testing in February 1953 led to the first patients being treated on September 7th that year. Meanwhile, work at the Stanford Microwave Laboratory led to the development of a 6-MV accelerator, which was installed at Stanford University Hospital, California, in 1956. Linac units quickly became favored devices for conventional fractionated radiotherapy. In February 1986, the first patient was treated in the United States at the Boston Brigham and Women’s Hospital.
Technical Process
The key component of SRS is the use of multiple beams. Each beam has very little effect on the tissue it passes through, but a targeted dose of radiation is delivered to the site where all the beams intersect. The intersection of the beams is designed to occur in the middle of the tumor; thus, giving maximum dosage while sparing the healthy surrounding tissues. Radiation delivered to the affected area causes tumors to shrink and blood vessels to close off over time following treatment, robbing the tumor of its blood supply. The whole treatment can be complete in one session; however, if the location of a tumor may be near a critical structure the radiation may be divided into fractions (typically 2-5 sessions). This is known as stereotactic radiotherapy (SRT). SRT is also useful in cases in which the tumor is larger than one inch in diameter. Delivering the radiation in a few fractions as opposed to one, can improve safety and allow the normal tissue to heal in between treatments. SRT relies more heavily on the different radiosensitivities of the target and the surrounding normal tissue than the total accumulated dose. If the tumor that is being treated is located in the body, and not in the central nervous system, the procedure can also be referred to as stereotactic body radiotherapy (SBRT).
SRS and SBRT rely on several similar technologies including 3D imaging and localization techniques that determine the exact coordinates of the target within the body, systems to immobilize and carefully position the patient, and highly focused radiation beams that converge on a malignancy. Additionally, image-guided radiation therapy (IGRT) is used to confirm the location of a tumor immediately before and possibly during the treatment.
SRS is amenable to the traditional workflow of radiation oncology. The physician is still overseeing the treatment and determining the most appropriate radiation dose. The medical physicist ensures the delivery of the precise dose of radiation. The dosimetrist devises a treatment plan and calculates the exposures and beam configuration to conformally treat the target to the prescribed dose and the radiation therapist positions the patient on the treatment table and operates the machine.
SRS and SBRT Uses
SRS is used to treat:
- many types of brain tumors including:
- benign and malignant
- primary and metastatic
- single and multiple
- residual tumor cells following surgery
- intracranial, orbital, and base-of-skull tumors
- arteriovenous malformations (AVMs), a tangle of expanded blood vessels that disrupts normal blood flow in the brain and sometimes bleeds.
- other neurological conditions
SBRT is currently used in treating malignant or benign small-to-medium size tumors in the body and common disease sites, including the:
- lung
- liver
- abdomen
- spine
- prostate
- head and neck
Types of Devices
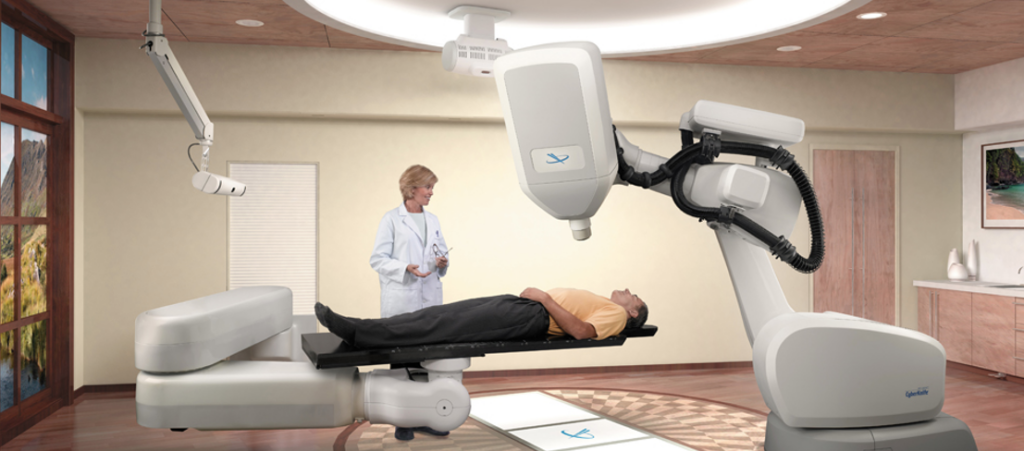
Gamma Knife and Linacs were briefly discussed in the history section. The key difference between the two is that Linacs utilizes photon beams to treat abnormalities. Contrastingly, Gamma Knife uses 192 or 201 small beams of gamma rays to target and treat malignancies. Gamma Knife machines are less common than LINAC machines and are used primarily for small to medium tumors and lesions in the brain associated with a variety of conditions. Furthermore, Gamma Knife is dedicated to radiosurgery while traditional Linacs are built for conventional fractionated radiotherapy and require additional technology and expertise to become dedicated radiosurgery tools. However, with new technology, dedicated radiosurgery Linacs have arisen in the last few decades, this includes the CyberKnife, a compact Linac mounted onto a robotic arm that moves around the patient and irradiates the tumor from a large set of fixed positions.
With the increased prevalence of Proton Therapy Centers, charged particle radiosurgery is the newest type of stereotactic radiosurgery and is available in only a few research centers in the U.S. Charged particle radiosurgery can be done in a single session using stereotactic radiosurgery, or it can use fractionated stereotactic radiotherapy.
Conclusion and Further Resources
SRS has allowed tumors that once were inoperable to be eradicated; thus, saving millions of lives over the years. Most centers are embracing this technology either as a boost following traditional radiotherapy or by itself. Furthermore, Brachytherapy, which consists of the precise placement of radioactive seeds directly into or next to the tumor, is being phased out due to the increasing prevalence and effectiveness of SBRT. Overall, SRS is improving patient outcomes and is a valuable tool in the radiation treatment arsenal.
For more resources on SRS, please check out the links below:
https://www.mayoclinic.org/tests-procedures/stereotactic-radiosurgery/about/pac-20384526
https://pubmed.ncbi.nlm.nih.gov/19751866/
https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Stereotactic-Radiosurgery
https://www.radiologyinfo.org/en/info/stereotactic